Magnesium sulfat: Perbedaan antara revisi
Tag: Suntingan visualeditor-wikitext |
Tag: halaman dengan galat kutipan Suntingan visualeditor-wikitext |
||
| Baris 114: | Baris 114: | ||
<references> |
<references> |
||
<ref name="fort2017">A. Dominic Fortes, Kevin S. Knight, and Ian G. Wood (2017): "Structure, thermal expansion and incompressibility of MgSO<sub>4</sub>·9H<sub>2</sub>O, its relationship to meridianiite (MgSO<sub>4</sub>·11H<sub>2</sub>O) and possible natural occurrences". ''Acta Crystallographica Section B: Structureal Science, Crystal Engineering and Materials'', volume 73, part 1, pages 47-64. {{doi|10.1107/S2052520616018266}}</ref> |
<ref name="fort2017">A. Dominic Fortes, Kevin S. Knight, and Ian G. Wood (2017): "Structure, thermal expansion and incompressibility of MgSO<sub>4</sub>·9H<sub>2</sub>O, its relationship to meridianiite (MgSO<sub>4</sub>·11H<sub>2</sub>O) and possible natural occurrences". ''Acta Crystallographica Section B: Structureal Science, Crystal Engineering and Materials'', volume 73, part 1, pages 47-64. {{doi|10.1107/S2052520616018266}}</ref> |
||
| ⚫ | <ref name="wsjdXXXX">{{cite news|url=https://www.wsj.com/articles/SB10001424052702303302504577327722133289222 |title=Quick Cures/Quack Cures: Is Epsom Worth Its Salt? |newspaper=[[The Wall Street Journal]]|date=9 April 2012 |access-date=15 June 2019 |url-status=live |archive-url=https://web.archive.org/web/20120412091100/https://online.wsj.com/article/SB10001424052702303302504577327722133289222.html |archive-date=12 April 2012 }}</ref> |
||
<ref name="buch2000">Industrial Inorganic Chemistry, Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner, John Wiley & Sons, 2d edition, 2000, {{ISBN|978-3-527-61333-5}}</ref> |
<ref name="buch2000">Industrial Inorganic Chemistry, Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner, John Wiley & Sons, 2d edition, 2000, {{ISBN|978-3-527-61333-5}}</ref> |
||
<ref name="pubchem">{{cite web|title=Pubchem: Magnesium Sulfate|url=https://pubchem.ncbi.nlm.nih.gov/compound/magnesium_sulfate|url-status=live|archive-url=https://web.archive.org/web/20210429232549/https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium-sulfate|archive-date=29 April 2021|access-date=13 September 2021|website=PubChem}}</ref> |
|||
| ⚫ | <ref name="odoc1995">{{cite journal | last1 = Odochian | first1 = Lucia | year = 1995 | title = Study of the nature of the crystallization water in some magnesium hydrates by thermal methods | url = http://resources.metapress.com/pdf-preview.axd?code=3q44807116q07153&size=largest | journal = Journal of Thermal Analysis and Calorimetry | volume = 45 | issue = 6 | pages = 1437–1448 | doi = 10.1007/BF02547437 | s2cid = 97855885 | access-date = 7 August 2010 | archive-url = https://web.archive.org/web/20110826021910/http://resources.metapress.com/pdf-preview.axd?code=3q44807116q07153&size=largest | archive-date = 26 August 2011 | url-status = dead }}</ref> |
||
<ref name="brew2016">{{Cite web|url=https://nationalhomebrew.com.au/beer/brewing-adjuncts-and-beer-enhancers/national-home-brew-magnesium-sulphate|title=Magnesium Sulphate|website=National Home Brew|archive-url=https://web.archive.org/web/20160801040013/https://nationalhomebrew.com.au/beer/brewing-adjuncts-and-beer-enhancers/national-home-brew-magnesium-sulphate|archive-date=1 August 2016|access-date=4 January 2019}}</ref> |
|||
| ⚫ | <ref name="fort2012">A. Dominic Fortes, Frank Browning, and Ian G. Wood (2012): "Cation substitution in synthetic meridianiite (MgSO<sub>4</sub>·11H<sub>2</sub>O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates". ''Physics and Chemistry of Minerals'', volume 39, issue, pages 419–441. {{doi|10.1007/s00269-012-0497-9}}</ref> |
||
<ref name="matsu2000">{{cite patent |inventor1-last= Matsuura |inventor1-first= Masaru |inventor2-last= Sasaki |inventor2-first= Masaoki |inventor3-last= Sasakib |inventor3-first= Jun |inventor4-last=Takeuchi |inventor4-first= Tomok |pubdate= 2000-03-28 |title= Process for producing packed tofu |country= US |number= 6042851 }}</ref> |
|||
<ref name="aqua2006">{{cite web|url = http://reefkeeping.com/issues/2006-07/rhf/index.php|title = Do-It-Yourself Magnesium Supplements for the Reef Aquarium|year = 2006|publisher = Reefkeeping|access-date = 2008-03-14|url-status = live|archive-url = https://web.archive.org/web/20080322114524/http://www.reefkeeping.com/issues/2006-07/rhf/index.php|archive-date = 22 March 2008}}</ref> |
|||
<ref name="pete2007">R. C. Peterson, W. Nelson, B. Madu, and H. F. Shurvell (2007): "Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars". ''American Mineralogist'', volume 92, issue 10, pages 1756–1759. {{doi|10.2138/am.2007.2668}}</ref> |
<ref name="pete2007">R. C. Peterson, W. Nelson, B. Madu, and H. F. Shurvell (2007): "Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars". ''American Mineralogist'', volume 92, issue 10, pages 1756–1759. {{doi|10.2138/am.2007.2668}}</ref> |
||
<ref name="sanc2015">{{cite journal | title = Ammoniated phyllosilicates with a likely outer Solar System origin on (1) Ceres |author1=M. C. De Sanctis |author2=E. Ammannito |author3=A. Raponi |author4=S. Marchi |author5=T. B. McCord |author6=H. Y. McSween |author7=F. Capaccioni |author8=M. T. Capria |author9=F. G. Carrozzo |author10=M. Ciarniello |author11=A. Longobardo |author12=F. Tosi |author13=S. Fonte |author14=M. Formisano |author15=A. Frigeri |author16=M. Giardino |author17=G. Magni |author18=E. Palomba |author19=D. Turrini |author20=F. Zambon |author21=J.-P. Combe |author22=W. Feldman |author23=R. Jaumann |author24=L. A. McFadden |author25=C. M. Pieters | journal = Nature | volume = 528 |issue=7581 | pages = 241–244 | date = 2015 | doi=10.1038/nature16172 | pmid=26659184|bibcode=2015Natur.528..241D |s2cid=1687271 |url=https://elib.dlr.de/101182/1/apf314pc_20151215_152518.pdf }}</ref> |
|||
<ref name="pete2006">{{cite journal |title=Alpersite (Mg,Cu)SO<sub>4</sub>·7H<sub>2</sub>O, a new mineral of the melanterite group, and cuprian pentahydrite: Their occurrence within mine waste |first1=Ronald C. |last1=Peterson |first2=Jane M. |last2=Hammarstrom |first3=Robert R |last3=Seal, II |journal=American Mineralogist |date=Feb 2006 |volume=91 |pages=261–269 |doi=10.2138/am.2006.1911 |issue=2–3|s2cid=56431885 }}</ref> |
<ref name="pete2006">{{cite journal |title=Alpersite (Mg,Cu)SO<sub>4</sub>·7H<sub>2</sub>O, a new mineral of the melanterite group, and cuprian pentahydrite: Their occurrence within mine waste |first1=Ronald C. |last1=Peterson |first2=Jane M. |last2=Hammarstrom |first3=Robert R |last3=Seal, II |journal=American Mineralogist |date=Feb 2006 |volume=91 |pages=261–269 |doi=10.2138/am.2006.1911 |issue=2–3|s2cid=56431885 }}</ref> |
||
| ⚫ | <ref name="odoc1995">{{cite journal | last1 = Odochian | first1 = Lucia | year = 1995 | title = Study of the nature of the crystallization water in some magnesium hydrates by thermal methods | url = http://resources.metapress.com/pdf-preview.axd?code=3q44807116q07153&size=largest | journal = Journal of Thermal Analysis and Calorimetry | volume = 45 | issue = 6 | pages = 1437–1448 | doi = 10.1007/BF02547437 | s2cid = 97855885 | access-date = 7 August 2010 | archive-url = https://web.archive.org/web/20110826021910/http://resources.metapress.com/pdf-preview.axd?code=3q44807116q07153&size=largest | archive-date = 26 August 2011 | url-status = dead }}</ref> |
||
<ref name="boots2018">{{cite web|title=Boots Magnesium Sulfate Paste B.P. - Patient Information Leaflet (PIL) - (eMC)|url=https://www.medicines.org.uk/emc/product/8327|website=www.medicines.org.uk|access-date=14 April 2018|language=en}}</ref> |
|||
<ref name="splinter">{{Cite web | url=http://www.tipking.co.uk/tip/5929.html | title=Removing a splinter with Magnesium Sulphate}}</ref> |
|||
<ref name="ingra2016">{{cite web|last1=Ingraham|first1=Paul|title=Does Epsom Salt Work? The science of Epsom salt bathing for recovery from muscle pain, soreness, or injury|url=https://www.painscience.com/articles/epsom-salts.php|website=Pain Science|access-date=29 August 2016|url-status=live|archive-url=https://web.archive.org/web/20160910223001/https://www.painscience.com/articles/epsom-salts.php|archive-date=10 September 2016}}</ref> |
|||
| ⚫ | <ref name="wsjdXXXX">{{cite news|url=https://www.wsj.com/articles/SB10001424052702303302504577327722133289222 |title=Quick Cures/Quack Cures: Is Epsom Worth Its Salt? |newspaper=[[The Wall Street Journal]]|date=9 April 2012 |access-date=15 June 2019 |url-status=live |archive-url=https://web.archive.org/web/20120412091100/https://online.wsj.com/article/SB10001424052702303302504577327722133289222.html |archive-date=12 April 2012 }}</ref> |
||
<ref name="rxmed2009">{{cite web|url=http://www.rxmed.com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20(General%20Monographs-%20M)/MAGNESIUM%20SULFATE.html|title=Pharmaceutical Information – Magnesium Sulfate|publisher=RxMed|access-date=2009-07-06|url-status=live|archive-url=https://web.archive.org/web/20090403180025/http://www.rxmed.com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20(General%20Monographs-%20M)/MAGNESIUM%20SULFATE.html|archive-date=3 April 2009}}</ref> |
|||
<ref name="heart2016">{{cite web|title=CPR and First Aid: Antiarrhythmic Drugs During and Immediately After Cardiac Arrest (section)|url=https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-7-adult-advanced-cardiovascular-life-support/?strue=1&id=5-3-2-1|publisher=American Heart Association|access-date=29 August 2016|quote=Previous ACLS guidelines addressed the use of magnesium in cardiac arrest with polymorphic ventricular tachycardia (ie, torsades de pointes) or suspected hypomagnesemia, and this has not been reevaluated in the 2015 Guidelines Update. These previous guidelines recommended defibrillation for termination of polymorphic VT (ie, torsades de pointes), followed by consideration of intravenous magnesium sulfate when secondary to a long QT interval.}}</ref> |
|||
<ref name="blitz2005">{{cite journal | vauthors = Blitz M, Blitz S, Hughes R, Diner B, Beasley R, Knopp J, Rowe BH | title = Aerosolized magnesium sulfate for acute asthma: a systematic review | journal = Chest | year = 2005 | volume = 128 | pages = 337–344 | doi = 10.1378/chest.128.1.337 | pmid = 16002955 | issue = 1 }}.</ref> |
|||
<ref name="duley2010">{{cite journal|last1=Duley|first1=L|last2=Gülmezoglu|first2=AM|last3=Henderson-Smart|first3=DJ|last4=Chou|first4=D|title=Magnesium sulphate and other anticonvulsants for women with pre-eclampsia.|journal=The Cochrane Database of Systematic Reviews|date=10 November 2010|issue=11|pages=CD000025|pmid=21069663|doi=10.1002/14651858.CD000025.pub2|pmc=7061250}}</ref> |
|||
| ⚫ | <ref name="fort2012">A. Dominic Fortes, Frank Browning, and Ian G. Wood (2012): "Cation substitution in synthetic meridianiite (MgSO<sub>4</sub>·11H<sub>2</sub>O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates". ''Physics and Chemistry of Minerals'', volume 39, issue, pages 419–441. {{doi|10.1007/s00269-012-0497-9}}</ref> |
||
<ref name="hcwood">{{cite book |last=Wood |first=H. C. |author-link=Horatio C. Wood Jr. |date=1877 |title=A Treatise on Therapeutics, Comprising Materia Medica and Toxicology, with Especial Reference to the Application of the Physiological Action of Drugs to Clinical Medicine |url=https://books.google.com/books?id=pjpHAQAAMAAJ&pg=PA34 |location=Philadelphia |publisher=[[J. B. Lippincott & Co.]] |page=34 |quote=The treatment of acute lead-poisoning consists in the evacuation of the stomach, if necessary, the exhibition of the sulphate of sodium or of magnesium, and the meeting of the indications as they arrive. The Epsom and Glauber's salts act as chemical antidotes, by precipitating the insoluble sulphate of lead, and also, if in excess, empty the bowel of the compound formed.}}</ref> |
|||
<ref name="Barker1945">{{cite journal |last=Barker |first=C. A. V. |date=January 1945 |title=Experience with Lead Poisoning |journal=Canadian Journal of Comparative Medicine and Veterinary Science |volume=9 |issue=1 |pages=6–8 |pmc=1660962 |pmid=17648099 |quote=Udall (1) suggests sodium citrate as of some value together with Epsom salts which will bring about a precipitation of the lead in the form of an insoluble compound. Nelson (3) reported a case that survived following the use of a 20% magnesium sulphate solution intravenously, subcutaneously and orally. McIntosh (5) has suggested that purgative doses of Epsom salts may be effective in combining with the lead and overcoming the toxicity.}}</ref> |
|||
<ref name="herriot">{{cite book |last=Herriot |first=James |author-link=James Herriot |date=1972 |title=All Creatures Great and Small |url=https://books.google.com/books?id=Hm-St_CUgsIC&pg=PA157 |location=New York |publisher=[[St. Martin's Press]] |page=157 |quote=The specific antidotes to metal poisoning had not been discovered and the only thing which sometimes did a bit of good was magnesium sulphate which caused the precipitation of insoluble lead sulphate. The homely term for magnesium sulphate is, of course, epsom salts. |isbn=0-312-08498-6}}</ref> |
|||
</references> |
</references> |
||
Revisi per 19 Desember 2021 07.13
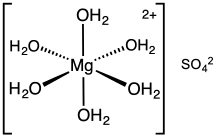 heksahidrat
| |
 Magnesium sulfat anhidrat
| |
 Epsomite (heptahidrat)
| |
| Nama | |
|---|---|
| Nama IUPAC
Magnesium sulfat
| |
| Nama lain
garam epsom (heptahidrat)
| |
| Penanda | |
| |
Model 3D (JSmol)
|
|
| 3DMet | {{{3DMet}}} |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| Nomor EC | |
PubChem CID
|
|
| Nomor RTECS | {{{value}}} |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Sifat | |
| MgSO4 | |
| Massa molar | 120.366 g/mol (anhidrat) 246.47 g/mol (heptahidrat) |
| Penampilan | padatan kristal putih |
| Bau | tak berbau |
| Densitas | 2.66 g/cm3 (anhidrat) 2.445 g/cm3 (monohidrat) 1.68 g/cm3 (heptahidrat) 1.512 g/cm3 (11-hidrat) |
| Titik lebur | anhidrat terdekomposisi pada 1124 °C monohidrat terdekomposisi pada 200 °C heptahidrat terdekomposisi pada 150 °C undekahidrat terdekomposisi pada 2 °C |
| anhidrat 26.9 g/100 mL (0 °C) 25.5 g/100 mL (20 °C) heptahidrat 71 g/100 mL (20 °C) | |
| Kelarutan | 1.16 g/100 mL (18 °C, eter) agak larut pada alkohol, gliserol tidak larut dalam aseton |
| Indeks bias (nD) | 1.523 (monohidrat) 1.433 (heptahidrat) |
| Struktur | |
| monoklinik (hidrat) | |
| Bahaya | |
| Senyawa terkait | |
Kation lainnya
|
Berilium sulfat Kalsium sulfat Stronsium sulfat Barium sulfat |
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |
| Referensi | |
Magnesium sulfat adalah senyawa kimia, suatu garam dengan rumus MgSO4, yang tersusun dari kation magnesium Mg2+ (20,19% b/b) dan anion sulfat SO2−4. Senyawa ini berbentuk kristal padat, yang larut dalam air tetapi tidak larut dalam etanol.
Magnesium sulfat biasanya dijumpai dalam bentuk terhidrasi MgSO4·nH2O, dengan nilai n yang beragam antara 1 dan 11. Bentuk terhidrasinya yang paling umum adalah magnesium sulfat heptahidrat MgSO4·7H2O, yang dikenal sebagai garam Epsom, yaitu bahan kimia rumah tangga multiguna, termasuk garam mandi.[1]
Penggunaan utama magnesium sulfat adalah pada bidang pertanian, untuk memperbaiki tanah yang kekurangan magnesium (suatu zat gizi tanaman esensial karena magnesium berperan penting dalam klorofil dan fotosintesis). Bentuk monohidratnya dipilih untuk penggunaan ini. Pada pertengahan tahun 1970an, produksi tahunannya mencapai 2,3 juta ton per tahun.[2] Bentuk anhidrat dan beberapa hidratnya terjadi di alam sebagai mineral, dan garamnya adalah komponen air yang penting dari beberapa mata air.
Hidrat
Magnesium sulfat dapat mengkristal sebagai hidratnya, antara lain:
- Anhidrat, MgSO4; tidak stabil di alam, terhidrasi membentuk epsomite.[3]
- Monohidrat, MgSO4·H2O; kieserite, monoklinik.[4]
- MgSO4·1.25H2O atau 8MgSO4·10H2O.[5]
- Dihidrat, MgSO4·2H2O; ortorombik.
- MgSO4·2.5H2O atau 2MgSO4·5H2O.[5]
- Trihidrat, MgSO4·3H2O.[5]
- Tetrahidrat, MgSO4·4H2O; starkeyite, monoklinik.[6]
- Pentahidrat, MgSO4·5H2O; pentahydrite, triklinik.[4]
- Heksahidrat, MgSO4·6H2O; hexahydrite, monoklinik.
- Heptahidrat, MgSO4·7H2O ("garam Epsom"); epsomite, ortorombik.[4]
- Eneahidrat, MgSO4·9H2O, monoklinik.[7]
- Dekahidrat, MgSO4·10H2O.[6]
- Undekahidrat, MgSO4·11H2O; meridianiite, triklinik.[6]
Hingga tahun 2017, keberadaan heksahidrat belum dikonfirmasi.[7]
Semua hidratnya akan membebaskan air kristalnya ketika dipanaskan. Di atas suhu 320 °C, hanya bentuk anhidratnya yang stabil. Ia terdekomposisi tanpa meleleh pada 1124 °C membentuk magnesium oksida (MgO) dan belerang trioksida (SO3).
Garam rangkap
Terdapat garam rangkap yang mengandung magnesium. Ada beberapa yang dikenal sebagai naterium magnesium sulfat dan kalium magnesium sulfat. Campuran tembaga-magnesium sulfat heptahidrat (Mg,Cu)SO4·7H2O baru-baru ini ditemukan terdapat pada tailing tambang dan mineral tersebut diberi nama alspersite.[8]
Lihat juga
Referensi
- ^ "Quick Cures/Quack Cures: Is Epsom Worth Its Salt?". The Wall Street Journal. 9 April 2012. Diarsipkan dari versi asli tanggal 12 April 2012. Diakses tanggal 15 June 2019.
- ^ Industrial Inorganic Chemistry, Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner, John Wiley & Sons, 2d edition, 2000, ISBN 978-3-527-61333-5
- ^ "Unnamed (Mg Sulphate)".
- ^ a b c Odochian, Lucia (1995). "Study of the nature of the crystallization water in some magnesium hydrates by thermal methods". Journal of Thermal Analysis and Calorimetry. 45 (6): 1437–1448. doi:10.1007/BF02547437. Diarsipkan dari versi asli tanggal 26 August 2011. Diakses tanggal 7 August 2010.
- ^ a b c A. Dominic Fortes, Frank Browning, and Ian G. Wood (2012): "Cation substitution in synthetic meridianiite (MgSO4·11H2O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates". Physics and Chemistry of Minerals, volume 39, issue, pages 419–441. DOI:10.1007/s00269-012-0497-9
- ^ a b c R. C. Peterson, W. Nelson, B. Madu, and H. F. Shurvell (2007): "Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars". American Mineralogist, volume 92, issue 10, pages 1756–1759. DOI:10.2138/am.2007.2668
- ^ a b A. Dominic Fortes, Kevin S. Knight, and Ian G. Wood (2017): "Structure, thermal expansion and incompressibility of MgSO4·9H2O, its relationship to meridianiite (MgSO4·11H2O) and possible natural occurrences". Acta Crystallographica Section B: Structureal Science, Crystal Engineering and Materials, volume 73, part 1, pages 47-64. DOI:10.1107/S2052520616018266
- ^ Peterson, Ronald C.; Hammarstrom, Jane M.; Seal, II, Robert R (Feb 2006). "Alpersite (Mg,Cu)SO4·7H2O, a new mineral of the melanterite group, and cuprian pentahydrite: Their occurrence within mine waste". American Mineralogist. 91 (2–3): 261–269. doi:10.2138/am.2006.1911.
Kesalahan pengutipan: Tag <ref> dengan nama "pubchem" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "brew2016" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "matsu2000" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "aqua2006" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "sanc2015" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "boots2018" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "splinter" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "ingra2016" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "rxmed2009" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "heart2016" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "blitz2005" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "duley2010" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "hcwood" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "Barker1945" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
Kesalahan pengutipan: Tag <ref> dengan nama "herriot" yang didefinisikan di <references> tidak digunakan pada teks sebelumnya.
