Kelimpahan alami unsur: Perbedaan antara revisi
k Robot: Perubahan kosmetika |
Tidak ada ringkasan suntingan |
||
| Baris 1: | Baris 1: | ||
[[Berkas:Relative abundance of elements.png|right|300px|Kelimpahan relatif unsur-unsur di Bumi]] |
|||
[[Berkas:Universe content pie chart.jpg|thumb|200px|Perkiraan proporsi [[materi]], [[materi gelap]] dan [[energi gelap]] di [[alam semesta]]. Hanya [[Fraksi massa (kimia)|fraksi]] [[massa]] dan [[energi]] di [[alam semesta]] berlabel "[[atom]]" yang disusun dalam [[unsur kimia]].]] |
|||
'''Kelimpahan alami unsur''' (''natural abundance'', NA) dalam [[ilmu kimia]] mengacu kepada kelimpahan [[isotop]]-isotop suatu [[unsur kimia]] yang secara alami dapat ditemukan di satu [[planet]]. [[Massa atom]] (rata-rata bobot) isotop-isotop ini adalah massa atom satu unsur kimia seperti yang ditulis dalam [[tabel periodik]]. Kelimpahan isotop berbeda dari planet ke planet, bahkan juga dari tempat ke tempat di [[Bumi]], namun tetap konstan setiap saat. |
|||
'''Kelimpahan unsur kimia''' adalah suatu ukuran keberadaan [[Unsur kimia|unsur]] relatif terhadap seluruh unsur yang ada di lingkungan. Kelimpahan diukur berdasarkan salah satu dari tiga cara berikut: berdasarkan [[fraksi massa (kimia)|fraksi massa]] (sama seperti fraksi berat); berdasarkan [[fraksi mol]] (fraksi jumlah atom, atau kadang-kadang fraksi molekul dalam gas ); atau berdasarkan [[fraksi volume]]. Fraksi volume adalah ukuran kelimpahan umum dalam campuran gas seperti atmosfer planet, dan memiliki nilai yang sama dengan fraksi mol melekul untuk campuran gas pada kerapatan dan tekanan relatif rendah, serta campuran [[gas ideal]]. Kebanyakan nilai kelimpahan dalam artikel ini dinyatakan sebagai fraksi massa.<!-- For example, the abundance of [[oxygen]] in pure [[water]] can be measured in two ways: the ''mass fraction'' is about 89%, because that is the fraction of water's mass which is oxygen. However, the ''mole-fraction'' is 33.3333...% because only 1 [[atom]] of 3 in water, H<sub>2</sub>O, is oxygen. |
|||
Sebagai contoh, [[uranium]] mempunyai tiga isotop yang dihasilkan secara alami: <sup>238</sup>U, <sup>235</sup>U dan <sup>234</sup>U. Kelimpahan alami masing-masing berkisar antara 99.2739 - 99.2752%, 0.7198 - 0.7202%, dan 0.0050 - 0.0059%.<ref>{{Citation | title = Uranium Isotopes | url = http://www.globalsecurity.org/wmd/intro/u-isotopes.htm | accessdate = 14 Maret 2012}}</ref> Apabila 100,000 atom uranium dianalisis, seseorang bisa menebak ada sekitar 99,275 atom <sup>238</sup>U, 720 atom <sup>235</sup>U, dan hanya 5 atau 6 atom <sup>234</sup>U. Ini karena <sup>238</sup>U jauh lebih stabil daripada <sup>235</sup>U atau <sup>234</sup>U, dan hal ini dapat dibuktikan dengan [[waktu paruh]] tiap isotop: 4.4468×10<sup>9</sup> tahun untuk <sup>238</sup>U berbanding dengan 7.038×10<sup>8</sup> bagi <sup>235</sup>U dan 245,500 tahun untuk <sup>234</sup>U. Namun, kelimpahan alami suatu isotop juga dipengaruhi oleh kemungkinan pembuatannnya dalam [[sintesis kimia|sintesis]] [[nuklir]] (seperti dalam kasus [[samarium]]; <sup>147</sup>Sm dan <sup>148</sup>Sm yang bentuk radioaktifnya lebih banyak di alam daripada <sup>144</sup>Sm yang stabil) dan juga oleh produksi suatu isotop melalui isotop alami radioaktif (seperti dalam kasus isotop [[timbal]] radiogenik). |
|||
As another example, looking at the ''mass-fraction'' abundance of hydrogen and helium in both the [[Universe]] as a whole and in the [[atmosphere]]s of [[Gas giant|gas-giant planet]]s such as [[Jupiter]], it is 74% for [[hydrogen]] and 23-25% for [[helium]]; while the ''(atomic) mole-fraction'' for hydrogen is 92%, and for helium is 8%, in these environments. Changing the given environment to [[Atmosphere of Jupiter|Jupiter's outer atmosphere]], where hydrogen is [[diatomic]] while helium is not, changes the ''molecular'' mole-fraction (fraction of total gas molecules), as well as the fraction of atmosphere by volume, of hydrogen to about 86%, and of helium to 13%.<ref group=Note>Below Jupiter's outer atmosphere, volume fractions are significantly different from mole fractions due to high temperatures (ionization and disproportionation) and high density where the [[Ideal Gas Law]] is inapplicable.</ref> |
|||
== Penyimpangan dari kelimpahan alami == |
|||
== Abundance of elements in the Universe == |
|||
{{wide image|Element-haeufigkeit.svg|1500px|alt=Kelimpahan relatif unsur di tata surya|Kelimpahan alami relatif unsur di tata surya. (skala logaritmik)}} |
|||
Dari studi tentang [[Matahari]] dan meteorit-meteorit primitif, kini telah diketahui bahwa [[sistem tata surya]] awalnya memiliki komposisi isotop yang hampir homogen. Deviasi dari rata-rata galaktik (yang berubah) yang disampel secara lokal sekitar waktu ketika pembakaran inti Matahari dimulai, dapat secara umum diperhitungkan dengan pemeringkatan massa dan sejumlah terbatas [[peluruhan radioaktif|peluruhan inti]] dan proses-proses [[transmutasi]].<ref>Robert N. Clayton (1978) Isotopic anomalies in the early solar system, ''Annual Review of Nuclear and Particle Science'' '''28''':501-522.</ref> Ada juga bukti penyuntikan isotop-isotop berjangka panjang pendek (kini hilang) dari ledakan supernova terdekat yang mungkin telah memulai runtuhnya [[nebula]] matahari.<ref>Ernst Zinner (2003) [http://www.sciencemag.org/cgi/content/summary/300/5617/265 An isotopic view of the early solar system], ''Science'' '''300''':5617, 265-267.</ref> Jadi, penyimpangan dari kelimpahan alami di bumi biasanya dinyatakan dalam bagian per seribu (permil atau ‰) karena kurang dari satu [[persen]] (%). |
|||
Satu pengecualian untuk ini terletak di dalam butiran pramatahari yang ditemukan dalam [[meteorit]] primitif. Ia telah melewati proses penghomogenan, dan sering membawa tanda inti proses-proses sintesis nuklir tertentu yang membentuk unsur-unsur butiran ini.<ref>Ernst Zinner (1998) Stellar nucleosynthesis and the isotopic composition of presolar grains from primitive meteorites, ''Annual Review of Earth and Planetary Sciences'' '''26''':147-188.</ref> Dalam materi ini, deviasi dari kelimpahan alami kadangkala diukur dalam faktor 100. |
|||
{| class="wikitable sortable" style="float:right" |
|||
|+Ten most common elements in the [[Milky Way Galaxy]] estimated spectroscopically<ref name="croswell">{{cite book | last = Croswell | first = Ken | title = Alchemy of the Heavens | publisher = Anchor |date=February 1996 | url = http://kencroswell.com/alchemy.html| isbn = 0-385-47214-5}}</ref> |
|||
|- |
|||
![[Atomic Number|Z]] !! Element !! colspan="2"|Mass fraction in parts per million |
|||
|- |
|||
| 1 || [[Hydrogen]] || style="text-align:right;"|739,000 || colspan="2"|71 × mass of oxygen (red bar) |
|||
|- |
|||
| 2 || [[Helium]] || style="text-align:right;"|240,000 || colspan="2"|23 × mass of oxygen (red bar) |
|||
|- |
|||
| 8 || [[Oxygen]] || style="text-align:right;"|{{bartable|10,400||0.01||background:red;}} |
|||
|- |
|||
| 6 || [[Carbon]] || style="text-align:right;"|{{bartable| 4,600||0.01}} |
|||
|- |
|||
| 10 || [[Neon]] || style="text-align:right;"|{{bartable| 1,340||0.01}} |
|||
|- |
|||
| 26 || [[Iron]] || style="text-align:right;"|{{bartable| 1,090||0.01}} |
|||
|- |
|||
| 7 || [[Nitrogen]] || style="text-align:right;"|{{bartable| 960||0.01}} |
|||
|- |
|||
| 14 || [[Silicon]] || style="text-align:right;"|{{bartable| 650||0.01}} |
|||
|- |
|||
| 12 || [[Magnesium]] || style="text-align:right;"|{{bartable| 580||0.01}} |
|||
|- |
|||
| 16 || [[Sulfur]] || style="text-align:right;"|{{bartable| 440||0.01}} |
|||
|} |
|||
== Lihat pula == |
|||
{{see also|Stellar population|Cosmochemistry|Astrochemistry}} |
|||
* [[Kelimpahan unsur]] |
|||
The elements – that is, ordinary ([[baryon]]ic) matter made of [[proton]]s, [[neutron]]s, and [[electron]]s, are only a small part of the content of the [[Universe]]. [[Observational cosmology|Cosmological observations]] suggest that only 4.6% of the universe's energy (including the mass contributed by energy, E = mc² ↔ m = E / c²) comprises the visible [[baryon]]ic [[matter]] that constitutes [[star]]s, [[planets]], and [[Life|living]] beings. The rest is made up of [[dark energy]] (72%) and [[dark matter]] (23%).<ref>[http://map.gsfc.nasa.gov/universe/uni_matter.html WMAP- Content of the Universe]</ref> These are forms of matter and energy believed to exist on the basis of [[scientific theory]] and observational [[Deductive reasoning|deduction]]s, but they have not been directly observed and their nature is not well understood. |
|||
Most standard (baryonic) matter is found in stars and interstellar clouds, in the form of atoms or [[ion]]s ([[plasma (physics)|plasma]]), although it can be found in degenerate forms in extreme astrophysical settings, such as the high densities inside [[white dwarf]]s and [[neutron star]]s. |
|||
[[Hydrogen]] is the most abundant element in the Universe; [[helium]] is second. However, after this, the rank of abundance does not continue to correspond to the [[atomic number]]; [[oxygen]] has abundance rank 3, but atomic number 8. All others are substantially less common. |
|||
The abundance of the lightest elements is well predicted by the [[Lambda-CDM model|standard cosmological model]], since they were mostly produced shortly (i.e., within a few hundred seconds) after the [[Big Bang]], in a process known as [[Big Bang nucleosynthesis]]. Heavier elements were mostly produced much later, inside of [[star]]s. |
|||
Hydrogen and helium are estimated to make up roughly 74% and 24% of all baryonic matter in the universe respectively. Despite comprising only a very small fraction of the universe, the remaining "heavy elements" can greatly influence astronomical phenomena. Only about 2% (by mass) of the [[Milky Way galaxy]]'s disk is composed of heavy elements. |
|||
These other elements are generated by stellar processes.<ref>{{cite journal|doi=10.1103/RevModPhys.28.53|title=Abundances of the Elements|date=1956|last1=Suess|first1=Hans|last2=Urey|first2=Harold|journal=Reviews of Modern Physics|volume=28|page=53|bibcode=1956RvMP...28...53S}}</ref><ref>{{cite journal|doi=10.1007/BF00172440|title=Abundances of the elements in the solar system|date=1973|last1=Cameron|first1=A.G.W.|journal=Space Science Reviews|volume=15|pages=121|bibcode=1973SSRv...15..121C}}</ref><ref>{{cite journal|doi=10.1016/0016-7037(82)90208-3|title=Solar-system abundances of the elements|date=1982|last1=Anders|first1=E|last2=Ebihara|first2=M|journal=Geochimica et Cosmochimica Acta|volume=46|page=2363|bibcode = 1982GeCoA..46.2363A|issue=11 }}</ref> In [[astronomy]], a "metal" is any element other than hydrogen or helium. This distinction is significant because hydrogen and helium are the only elements that were produced in significant quantities in the Big Bang. Thus, the [[metallicity]] of a [[galaxy]] or other object is an indication of stellar activity, after the Big Bang. |
|||
The following graph (note log scale) shows abundance of elements in our solar system. The table shows the twelve most common elements in our galaxy (estimated spectroscopically), as measured in parts per million, by mass.<ref name="croswell"/> |
|||
Nearby galaxies that have evolved along similar lines have a corresponding enrichment of elements heavier than hydrogen and helium. The more distant galaxies are being viewed as they appeared in the past, so their abundances of elements appear closer to the primordial mixture. Since physical laws and processes are uniform throughout the universe, however, it is expected that these galaxies will likewise have evolved similar abundances of elements. |
|||
[[Image:SolarSystemAbundances.png|thumb|right|800px|Estimated abundances of the chemical '''elements in the Solar system'''. Hydrogen and helium are most common, from the [[Big Bang]]. The next three elements (Li, Be, B) are rare because they are poorly synthesized in the Big Bang and also in stars. The two general trends in the remaining stellar-produced elements are: (1) an alternation of abundance in elements as they have even or odd atomic numbers (the [[Oddo-Harkins rule]]), and (2) a general decrease in abundance, as elements become heavier. Iron is especially common because it represents the minimum energy nuclide that can be made by fusion of helium in supernovae.]] |
|||
The abundance of elements in the [[Solar System]] (see graph) is in keeping with their origin from the [[Big Bang]] and [[nucleosynthesis]] in a number of progenitor [[supernova]] stars. Very abundant hydrogen and helium are products of the Big Bang, while the next three elements are rare since they had little time to form in the Big Bang and are not made in stars (they are, however, produced in small quantities by breakup of heavier elements in interstellar dust, as a result of impact by [[cosmic ray]]s). |
|||
Beginning with carbon, elements have been produced in stars by buildup from [[alpha particle]]s (helium nuclei), resulting in an alternatingly larger abundance of elements with even atomic numbers (these are also more stable). The effect of odd-numbered chemical elements generally being more rare in the universe was empirically noticed in 1914, and is known as the [[Oddo-Harkins rule]]. |
|||
[[File:Nucleosynthesis periodic table.svg|thumb|500px|Periodic table showing the cosmogenic origin of each element]] |
|||
'''Cosmogenesis:''' In general, such elements up to iron are made in large stars in the process of becoming [[supernova]]e. [[Iron-56]] is particularly common, since it is the most stable element that can easily be made from alpha particles (being a product of decay of radioactive [[nickel-56]], ultimately made from 14 helium nuclei). Elements heavier than iron are made in energy-absorbing processes in large stars, and their abundance in the universe (and on Earth) generally decreases with increasing atomic number. |
|||
{{clear}} |
|||
{| class="wikitable sortable" style="float:right" |
|||
|+ Most abundant isotopes in the [[Solar System]]<ref>{{cite book |first=David |last=Arnett |date=1996 |title=Supernovae and Nucleosynthesis |edition=First |publisher=Princeton University Press |location=Princeton, New Jersey|url=http://books.google.com/?id=PXGWGnPPo0gC&printsec=frontcove |isbn=0-691-01147-8 |oclc=33162440}}</ref> |
|||
![[Isotope]] |
|||
![[Atomic Mass|A]] |
|||
!width="1"|<!-- Make column as narrow as possible --> Mass fraction in parts per million |
|||
!width="1"|<!-- Make column as narrow as possible --> Atom fraction in parts per million |
|||
|- |
|||
| [[Hydrogen-1]] ||1||style="text-align:right"|705,700 ||style="text-align:right"| 909,964 |
|||
|- |
|||
| [[Helium-4]] ||4||style="text-align:right"|275,200 ||style="text-align:right"| 88,714 |
|||
|- |
|||
| [[Oxygen-16]] ||16||style="text-align:right"|5,920 ||style="text-align:right"| 477 |
|||
|- |
|||
| [[Carbon-12]] ||12||style="text-align:right"|3,032 ||style="text-align:right"| 326 |
|||
|- |
|||
| [[Nitrogen-14]] ||14||style="text-align:right"|1,105 ||style="text-align:right"| 102 |
|||
|- |
|||
| [[Neon-20]] ||20||style="text-align:right"|1,548 ||style="text-align:right"| 100 |
|||
|- |
|||
| colspan="4" | [[Image:spacer.gif|1px]] |
|||
|- |
|||
| colspan="2" | '''''Other isotopes:''''' ||style="text-align:right"|3,879 ||style="text-align:right"| 149 |
|||
|- |
|||
| [[Silicon-28]] ||28||style="text-align:right"|653 ||style="text-align:right"| 30 |
|||
|- |
|||
| [[Magnesium-24]] ||24||style="text-align:right"|513 ||style="text-align:right"| 28 |
|||
|- |
|||
| [[Iron-56]] ||56||style="text-align:right"|1,169 ||style="text-align:right"| 27 |
|||
|- |
|||
| [[Sulfur-32]] ||32||style="text-align:right"|396 ||style="text-align:right"| 16 |
|||
|- |
|||
| [[Helium-3]] ||3||style="text-align:right"|35 ||style="text-align:right"| 15 |
|||
|- |
|||
| [[Hydrogen-2]] ||2||style="text-align:right"|23 ||style="text-align:right"| 15 |
|||
|- |
|||
| [[Neon-22]] ||22||style="text-align:right"|208 ||style="text-align:right"| 12 |
|||
|- |
|||
| [[Magnesium-26]] ||26||style="text-align:right"|79 ||style="text-align:right"| 4 |
|||
|- |
|||
| [[Carbon-13]] ||13||style="text-align:right"|37 ||style="text-align:right"| 4 |
|||
|- |
|||
| [[Magnesium-25]] ||25||style="text-align:right"|69 ||style="text-align:right"| 4 |
|||
|- |
|||
| [[Aluminum-27]] ||27||style="text-align:right"|58 ||style="text-align:right"| 3 |
|||
|- |
|||
| [[Argon-36]] ||36||style="text-align:right"|77 ||style="text-align:right"| 3 |
|||
|- |
|||
| [[Calcium-40]] ||40||style="text-align:right"|60 ||style="text-align:right"| 2 |
|||
|- |
|||
| [[Sodium-23]] ||23||style="text-align:right"|33 ||style="text-align:right"| 2 |
|||
|- |
|||
| [[Iron-54]] ||54||style="text-align:right"|72 ||style="text-align:right"| 2 |
|||
|- |
|||
| [[Silicon-29]] ||29||style="text-align:right"|34 ||style="text-align:right"| 2 |
|||
|- |
|||
| [[Nickel-58]] ||58||style="text-align:right"|49 ||style="text-align:right"| 1 |
|||
|- |
|||
| [[Silicon-30]] ||30||style="text-align:right"|23 ||style="text-align:right"| 1 |
|||
|- |
|||
| [[Iron-57]] ||57||style="text-align:right"|28 ||style="text-align:right"| 1 |
|||
|} |
|||
===Elemental abundance and nuclear binding energy=== |
|||
Loose correlations have been observed between estimated elemental abundances in the universe and the [[Nuclear binding energy#Nuclear binding energy curve|nuclear binding energy curve]]. Roughly speaking, the relative stability of various atomic isotopes has exerted a strong influence on the relative abundance of elements formed in the [[Big Bang]], and during the development of the universe thereafter. |
|||
<ref name=Bell01>{{cite book|last=Bell|first=Jerry A.|title=Chemistry: a project of the American Chemical Society|date=2005|publisher=Freeman|location=New York [u.a.]|isbn=978-0-7167-3126-9|pages=191–193|author2=GenChem Editorial/Writing Team|chapter=Chapter 3: Origin of Atoms|quote=Correlations between abundance and nuclear binding energy [Subsection title]}}</ref> |
|||
See the article about [[nucleosynthesis]] for the explanation on how certain [[nuclear fusion]] processes in stars (such as [[carbon-burning process|carbon burning]], etc.) create the elements heavier than hydrogen and helium. |
|||
A further observed peculiarity is the jagged alternation between relative abundance and scarcity of adjacent atomic numbers in the elemental abundance curve, and a similar pattern of energy levels in the nuclear binding energy curve. This alternation is caused by the higher relative [[binding energy]] (corresponding to relative stability) of even atomic numbers compared to odd atomic numbers, and is explained by the [[Pauli Exclusion Principle]].<ref name=Bell02>{{cite book|last=Bell|first=Jerry A.|title=Chemistry: a project of the American Chemical Society|date=2005|publisher=Freeman|location=New York [u.a.]|isbn=978-0-7167-3126-9|page=192|author2=GenChem Editorial/Writing Team|chapter=Chapter 3: Origin of Atoms|quote=The higher abundance of elements with even atomic numbers [Subsection title]}}</ref> |
|||
The [[semi-empirical mass formula]] (SEMF), also called '''Weizsäcker's formula''' or the '''Bethe-Weizsäcker mass formula''', gives a theoretical explanation of the overall shape of the curve of nuclear binding energy.<ref name=Bailey>{{cite web|last=Bailey|first=David|title=Semi-empirical Nuclear Mass Formula|url=http://www.upscale.utoronto.ca/GeneralInterest/DBailey/SubAtomic/Lectures/LectF25/Lect25.htm|work=PHY357: Strings & Binding Energy|publisher=University of Toronto|accessdate=2011-03-31}}</ref> |
|||
{{clear}} |
|||
== Abundance of elements in the Earth == |
|||
{{see also|Earth#Chemical composition}} |
|||
The [[Earth]] formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the [[formation and evolution of the solar system]]. In turn, the [[history of Earth|natural history of the Earth]] caused parts of this planet to have differing concentrations of the elements. |
|||
The mass of the Earth is approximately 5.98{{e|24}} kg. In bulk, by mass, it is composed mostly of [[iron]] (32.1%), [[oxygen]] (30.1%), [[silicon]] (15.1%), [[magnesium]] (13.9%), [[sulfur]] (2.9%), [[nickel]] (1.8%), [[calcium]] (1.5%), and [[aluminium]] (1.4%); with the remaining 1.2% consisting of trace amounts of other elements.<ref name=pnas71_12_6973>{{cite journal | author=Morgan, J. W. | author2=Anders, E. | title=Chemical composition of Earth, Venus, and Mercury | journal=Proceedings of the National Academy of Sciences | date=1980 | volume=77 | issue=12 | pages=6973–6977 | doi=10.1073/pnas.77.12.6973 | pmid=16592930 | pmc=350422 |bibcode = 1980PNAS...77.6973M }}</ref> |
|||
The bulk composition of the Earth by elemental-mass is roughly similar to the gross composition of the solar system, with the major differences being that Earth is missing a great deal of the volatile elements hydrogen, helium, neon, and nitrogen, as well as carbon which has been lost as volatile hydrocarbons. The remaining elemental composition is roughly typical of the "rocky" inner planets, which formed in the thermal zone where solar heat drove volatile compounds into space. The Earth retains oxygen as the second-largest component of its mass (and largest atomic-fraction), mainly from this element being retained in [[silicate minerals]] which have a very high melting point and low vapor pressure. |
|||
=== Earth's detailed bulk (total) elemental abundance in table form === |
|||
Click "show" at right, to show more numerical values in a full table. Note that these are ordered by atom-fraction abundance (right-most column), not mass-abundance. |
|||
{| class="wikitable sortable collapsible collapsed" style="font-size:75%; float:right; margin-right: 0; margin-left:1em; text-align:right" |
|||
|- |
|||
! Number |
|||
! Name |
|||
! Symbol |
|||
! ppm (μg/g) |
|||
! ppb (atoms){{Citation needed|date=September 2011}} |
|||
|- |
|||
|8 || oxygen || O || 297000 || 482,000,000 |
|||
|- |
|||
|12 || magnesium || Mg || 154000 || 164,000,000 |
|||
|- |
|||
|14 || silicon || Si || 161000 || 150,000,000 |
|||
|- |
|||
|26 || iron || Fe || 319000 || 148,000,000 |
|||
|- |
|||
|13 || aluminum || Al || 15900 || 15,300,000 |
|||
|- |
|||
|20 || calcium || Ca || 17100 || 11,100,000 |
|||
|- |
|||
|28 || nickel || Ni || 18220 || 8,010,000 |
|||
|- |
|||
|1 || hydrogen || H || 260 || 6,700,000 |
|||
|- |
|||
|16 || sulfur || S || 6350 || 5,150,000 |
|||
|- |
|||
|24 || chromium || Cr || 4700 || 2,300,000 |
|||
|- |
|||
|11 || sodium || Na || 1800 || 2,000,000 |
|||
|- |
|||
|6 || carbon || C || 730 || 1,600,000 |
|||
|- |
|||
|15 || phosphorus || P || 1210 || 1,020,000 |
|||
|- |
|||
|25 || manganese || Mn || 1700 || 800,000 |
|||
|- |
|||
|22 || titanium || Ti || 810 || 440,000 |
|||
|- |
|||
|27 || cobalt || Co || 880 || 390,000 |
|||
|- |
|||
|19 || potassium || K || 160 || 110,000 |
|||
|- |
|||
|17 || chlorine || Cl || 76 || 56,000 |
|||
|- |
|||
|23 || vanadium || V || 105 || 53,600 |
|||
|- |
|||
|7 || nitrogen || N || 25 || 46,000 |
|||
|- |
|||
|29 || copper || Cu || 60 || 25,000 |
|||
|- |
|||
|30 || zinc || Zn || 40 || 16,000 |
|||
|- |
|||
|9 || fluorine || F || 10 || 14,000 |
|||
|- |
|||
|21 || scandium || Sc || 11 || 6,300 |
|||
|- |
|||
|3 || lithium || Li || 1.10 || 4,100 |
|||
|- |
|||
|38 || strontium || Sr || 13 || 3,900 |
|||
|- |
|||
|32 || germanium || Ge || 7.00 || 2,500 |
|||
|- |
|||
|40 || zirconium || Zr || 7.10 || 2,000 |
|||
|- |
|||
|31 || gallium || Ga || 3.00 || 1,000 |
|||
|- |
|||
|34 || selenium || Se || 2.70 || 890 |
|||
|- |
|||
|56 || barium || Ba || 4.50 || 850 |
|||
|- |
|||
|39 || yttrium || Y || 2.90 || 850 |
|||
|- |
|||
|33 || arsenic || As || 1.70 || 590 |
|||
|- |
|||
|5 || boron || B || 0.20 || 480 |
|||
|- |
|||
|42 || molybdenum || Mo || 1.70 || 460 |
|||
|- |
|||
|44 || ruthenium || Ru || 1.30 || 330 |
|||
|- |
|||
|78 || platinum || Pt || 1.90 || 250 |
|||
|- |
|||
|46 || palladium || Pd || 1.00 || 240 |
|||
|- |
|||
|58 || cerium || Ce || 1.13 || 210 |
|||
|- |
|||
|60 || neodymium || Nd || 0.84 || 150 |
|||
|- |
|||
|4 || beryllium || Be || 0.05 || 140 |
|||
|- |
|||
|41 || niobium || Nb || 0.44 || 120 |
|||
|- |
|||
|76 || osmium || Os || 0.90 || 120 |
|||
|- |
|||
|77 || iridium || Ir || 0.90 || 120 |
|||
|- |
|||
|37 || rubidium || Rb || 0.40 || 120 |
|||
|- |
|||
|35 || bromine || Br || 0.30 || 97 |
|||
|- |
|||
|57 || lanthanum || La || 0.44 || 82 |
|||
|- |
|||
|66 || dysprosium || Dy || 0.46 || 74 |
|||
|- |
|||
|64 || gadolinium || Gd || 0.37 || 61 |
|||
|- |
|||
|52 || tellurium || Te || 0.30 || 61 |
|||
|- |
|||
|45 || rhodium || Rh || 0.24 || 61 |
|||
|- |
|||
|50 || tin || Sn || 0.25 || 55 |
|||
|- |
|||
|62 || samarium || Sm || 0.27 || 47 |
|||
|- |
|||
|68 || erbium || Er || 0.30 || 47 |
|||
|- |
|||
|70 || ytterbium || Yb || 0.30 || 45 |
|||
|- |
|||
|59 || praseodymium || Pr || 0.17 || 31 |
|||
|- |
|||
|82 || lead || Pb || 0.23 || 29 |
|||
|- |
|||
|72 || hafnium || Hf || 0.19 || 28 |
|||
|- |
|||
|74 || tungsten || W || 0.17 || 24 |
|||
|- |
|||
|79 || gold || Au || 0.16 || 21 |
|||
|- |
|||
|48 || cadmium || Cd || 0.08 || 18 |
|||
|- |
|||
|63 || europium || Eu || 0.10 || 17 |
|||
|- |
|||
|67 || holmium || Ho || 0.10 || 16 |
|||
|- |
|||
|47 || silver || Ag || 0.05 || 12 |
|||
|- |
|||
|65 || terbium || Tb || 0.07 || 11 |
|||
|- |
|||
|51 || antimony || Sb || 0.05 || 11 |
|||
|- |
|||
|75 || rhenium || Re || 0.08 || 10 |
|||
|- |
|||
|53 || iodine || I || 0.05 || 10 |
|||
|- |
|||
|69 || thulium || Tm || 0.05 || 7 |
|||
|- |
|||
|55 || cesium || Cs || 0.04 || 7 |
|||
|- |
|||
|71 || lutetium || Lu || 0.05 || 7 |
|||
|- |
|||
|90 || thorium || Th || 0.06 || 6 |
|||
|- |
|||
|73 || tantalum || Ta || 0.03 || 4 |
|||
|- |
|||
|80 || mercury || Hg || 0.02 || 3 |
|||
|- |
|||
|92 || uranium || U || 0.02 || 2 |
|||
|- |
|||
|49 || indium || In || 0.01 || 2 |
|||
|- |
|||
|81 || thallium || Tl || 0.01 || 2 |
|||
|- |
|||
|83 || bismuth || Bi || 0.01 || 1 |
|||
|} |
|||
An estimate<ref>William F McDonough [http://quake.mit.edu/hilstgroup/CoreMantle/EarthCompo.pdf The composition of the Earth]. quake.mit.edu</ref> of the elemental abundances in the total mass of the Earth. Note that numbers are estimates, and they will vary depending on source and method of estimation. Order of magnitude of data can roughly be relied upon. |
|||
ppb (atoms) is parts per billion, meaning that is the number of atoms of a given element in every billion atoms in the Earth. |
|||
=== Earth's crustal elemental abundance === |
|||
{{main|Abundance of elements in Earth's crust}} |
|||
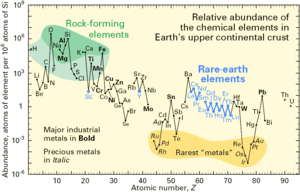
[[File:Elemental abundances.svg|thumb|400px|left|Abundance (atom fraction) of the chemical elements in Earth's upper continental crust as a function of atomic number. The rarest elements in the crust (shown in yellow) are the most dense. They were further rarefied in the crust by being siderophile (iron-loving) elements, in the [[Goldschmidt classification]] of elements. Siderophiles were depleted by being relocated into the Earth's core. Their abundance in [[meteoroid]] materials is relatively higher. Additionally, tellurium and selenium have been depleted from the crust due to formation of volatile hydrides.]] |
|||
The mass-abundance of the nine most abundant elements in the Earth's crust (see main article above) is approximately: oxygen 46%, silicon 28%, aluminum 8.2%, iron 5.6%, calcium 4.2%, sodium 2.5%, magnesium 2.4%, potassium, 2.0%, and titanium 0.61%. Other elements occur at less than 0.15%. |
|||
The graph at left illustrates the relative atomic-abundance of the chemical elements in Earth's upper continental crust, which is relatively accessible for measurements and estimation. |
|||
Many of the elements shown in the graph are classified into (partially overlapping) categories: |
|||
# rock-forming elements (major elements in green field, and minor elements in light green field); |
|||
# [[rare earth element]]s (lanthanides, La-Lu, and Y; labeled in blue); |
|||
# major industrial metals (global production >~3×10<sup>7</sup> kg/year; labeled in red); |
|||
# [[precious metal]]s (labeled in purple); |
|||
# the nine rarest "metals" — the six [[platinum group]] elements plus [[Gold|Au]], [[Rhenium|Re]], and [[Tellurium|Te]] (a metalloid) — in the yellow field. |
|||
Note that there are two breaks where the unstable elements [[technetium]] (atomic number: 43) and [[promethium]] (atomic number: 61) would be. These are both extremely rare, since on Earth they are only produced through the [[nuclear fission|spontaneous fission]] of very heavy [[radioactive decay|radioactive]] elements (for example, [[uranium]], [[thorium]], or the trace amounts of [[plutonium]] that exist in uranium ores), or by the interaction of certain other elements with [[cosmic ray]]s. Both of the first two of these elements have been identified spectroscopically in the atmospheres of stars, where they are produced by ongoing nucleosynthetic processes. There are also breaks where the six [[noble gas]]es would be, since they are not chemically bound in the Earth's crust, and they are only generated by decay chains from radioactive elements and are therefore extremely rare there. The twelve naturally occurring very rare, highly radioactive elements ([[polonium]], [[astatine]], [[francium]], [[radium]], [[actinium]], [[protactinium]], [[neptunium]], [[plutonium]], [[americium]], [[curium]], [[berkelium]], and [[californium]]) are not included, since any of these elements that were present at the formation of the Earth have decayed away eons ago, and their quantity today is negligible and is only produced from the [[radioactive decay]] of uranium and thorium. |
|||
[[Oxygen]] and [[silicon]] are notably quite common elements in the crust. They have frequently combined with each other to form common [[silicate mineral]]s. |
|||
==== Crustal rare-earth elemental abundance ==== |
|||
"Rare" earth elements is a historical misnomer. The persistence of the term reflects unfamiliarity rather than true rarity. The more abundant [[rare earth element]]s are each similar in crustal concentration to commonplace industrial metals such as chromium, nickel, copper, zinc, molybdenum, tin, tungsten, or lead. The two least abundant rare earth elements ([[thulium]] and [[lutetium]]) are nearly 200 times more common than gold. However, in contrast to the ordinary base and precious metals, rare earth elements have very little tendency to become concentrated in exploitable ore deposits. Consequently, most of the world's supply of rare earth elements comes from only a handful of sources. Furthermore, the rare earth metals are all quite chemically similar to each other, and they are thus quite difficult to separate into quantities of the pure elements. |
|||
Differences in abundances of individual rare earth elements in the upper continental crust of the Earth represent the superposition of two effects, one nuclear and one geochemical. First, the rare earth elements with even atomic numbers (<sub>58</sub>Ce, <sub>60</sub>Nd, ...) have greater cosmic and terrestrial abundances than the adjacent rare earth elements with odd atomic numbers (<sub>57</sub>La, <sub>59</sub>Pr, ...). Second, the lighter rare earth elements are more incompatible (because they have larger ionic radii) and therefore more strongly concentrated in the continental crust than the heavier rare earth elements. In most rare earth ore deposits, the first four rare earth elements – [[lanthanum]], [[cerium]], [[praseodymium]], and [[neodymium]] – constitute 80% to 99% of the total amount of rare earth metal that can be found in the ore. |
|||
{{Clear}} |
|||
=== Earth's mantle elemental abundance === |
|||
{{main|Mantle (geology)}} |
|||
The mass-abundance of the eight most abundant elements in the Earth's crust (see main article above) is approximately: oxygen 45%, magnesium 23%, silicon 22%, iron 5.8%, calcium 2.3%, aluminum 2.2%, sodium 0.3%, potassium 0.3%. |
|||
The mantle differs in elemental composition from the crust in having a great deal more magnesium and significantly more iron, while having much less aluminum and sodium. |
|||
=== Earth's core elemental abundance === |
|||
Due to [[mass segregation]], the core of the Earth is believed to be primarily composed of iron (88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.<ref name=pnas71_12_6973/> |
|||
=== Oceanic elemental abundance === |
|||
{| class="wikitable" style="float: left" |
|||
|+ Earth's ocean water elemental abundance |
|||
|- |
|||
! Element !! colspan="2"|Proportion (by mass) |
|||
|- |
|||
| [[Oxygen]] || {{bartable| 85.84|%}} |
|||
|- |
|||
| [[Hydrogen]] || {{bartable| 10.82|%}} |
|||
|- |
|||
| [[Chlorine]] || {{bartable| 1.94|%}} |
|||
|- |
|||
| [[Sodium]] || {{bartable| 1.08|%}} |
|||
|- |
|||
| [[Magnesium]] || {{bartable|0.1292|%}} |
|||
|- |
|||
| [[Sulfur]] || {{bartable| 0.091|%}} |
|||
|- |
|||
| [[Calcium]] || {{bartable| 0.04|%}} |
|||
|- |
|||
| [[Potassium]] || {{bartable| 0.04|%}} |
|||
|- |
|||
| [[Bromine]] || {{bartable|0.0067|%}} |
|||
|- |
|||
| [[Carbon]] || {{bartable|0.0028|%}} |
|||
|} |
|||
{{for|a complete list of the abundance of elements in the ocean|Abundances of the elements (data page)#Sea water}} |
|||
{{clear}} |
|||
=== Atmospheric elemental abundance === |
|||
The order of elements by volume-fraction (which is approximately molecular mole-fraction) in the [[Earth's atmosphere|atmosphere]] is [[nitrogen]] (78.1%), [[oxygen]] (20.9%),<ref name="NYT-20131003">{{cite news |last=Zimmer |first=Carl |authorlink=Carl Zimmer |title=Earth’s Oxygen: A Mystery Easy to Take for Granted |url=http://www.nytimes.com/2013/10/03/science/earths-oxygen-a-mystery-easy-to-take-for-granted.html |date=3 October 2013 |work=[[New York Times]] |accessdate=3 October 2013 }}</ref> [[argon]] (0.96%), followed by (in uncertain order) carbon and hydrogen because water vapor and carbon dioxide, which represent most of these two elements in the air, are variable components. Sulfur, phosphorus, and all other elements are present in significantly lower proportions. |
|||
According to the abundance curve graph (above right), argon, a significant if not major component of the atmosphere, does not appear in the crust at all. This is because the atmosphere has a far smaller mass than the crust, so argon remaining in the crust contributes little to mass-fraction there, while at the same time buildup of argon in the atmosphere has become large enough to be significant. <!--it's not that argon can't stay in the crust due to being inert. Helium stays in the crust well enough! And there is argon trapped in rocks or K-Ar dating would not work. Instead, the reason is the one given. |
|||
{{Clear}} |
|||
=== Abundances of elements in urban soils === |
|||
''For a complete list of the abundance of elements in urban soils, see [[Abundances of the elements (data page)#Urban soils]].'' |
|||
==== Reasons for establishing ==== |
|||
In the time of life existence, or at least in the time of the existence of [[human]] beings, the abundances of chemical elements within the Earth's crust have not been changed dramatically due to migration and concentration processes except the [[radioactive element]]s and their [[decay products]] and also [[noble gases]]. However, significant changes took place in the distribution of chemical elements. But within the [[biosphere]] not only the distribution, but also the abundances of elements have changed during the last centuries. |
|||
The rate of a number of geochemical changes taking place during the last decades in the [[biosphere]] has become catastrophically high. Such changes are often connected with [[Human impact on the environment|human activities]]. To study these changes and to make better informed decisions on diminishing their adverse impact on living organisms, and especially on people, it is necessary to estimate the contemporary abundances of chemical elements in geochemical systems susceptible to the highest anthropogenic impact and having a significant effect on the development and existence of living organisms. One of such systems is the [[soil]] of [[Urban area|urban landscapes]]. [[Human settlements|Settlements]] occupy less than 10% of the [[Land mass|land area]], but virtually the entire population of the planet lives within them. The main deposing medium in cities is soil, which ecological and geochemical conditions largely determine the life safety of citizens. So that, one of the priority tasks of the environmental geochemistry is to establish the average contents (abundances) of chemical elements in the soils of settlements. |
|||
==== Methods and results ==== |
|||
[[File:Half-logarithm graph.jpg|thumb|600px|left|framed|left|'''The half-logarithm graph of the abundances of chemical elements in urban soils.''' ([http://www.sciencedirect.com/science/article/pii/S0375674214002866 Alekseenko and Alekseenko, 2014]) Chemical elements are distributed extremely irregularly in urban soils, what is also typical for the Earth's crust. Nine elements (O, Si, Ca, C, Al, Fe, H, K, N) make the 97.68% of the considering geochemical system (urban soils). These elements and also Zn, Sr, Zr, Ba, and Pb essentially prevail over the trend line. Part of them could be considered as “inherited” from the concentrations in the Earth's crust; another part is explained as a result of intensive technogenic activity in the cities. ]] |
|||
The geochemical properties of urban soils from more than 300 cities in [[Europe]], [[Asia]], [[Africa]], [[Australia]], and [[Americas|America]] were evaluated.<ref>{{cite journal|author1=Vladimir Alekseenko|author2=Alexey Alekseenko|title=The abundances of chemical elements in urban soils|journal=Journal of Geochemical Exploration|date=2014|volume=147|pages=245–249|doi=10.1016/j.gexplo.2014.08.003|publisher=Elsevier B.V.|issn=0375-6742}}</ref> In each settlement samples were collected uniformly throughout the territory, covering residential, industrial, recreational and other urban areas. The sampling was carried out directly from the soil surface and specifically traversed pits, ditches and wells from the upper soil horizon. The number of samples in each locality ranged from 30 to 1000. The published data and the materials kindly provided by a number of geochemists were also incorporated into the research. Considering the great importance of the defined contents, quantitative and quantitative emission [[Mass spectrometry|spectral]], [[Gravimetric analysis|gravimetric]], [[X-ray fluorescence analysis|X-ray fluorescence]], and partly [[Neutron activation analysis|neutron activation]] analyses were carried out in parallel approximately in the samples. In a volume of 3–5% of the total number of samples, sampling and analyses of the inner and external controls were conducted. Calculation of [[random errors]] and [[systematic errors]] allowed to consider the sampling and analytical laboratory work as good. |
|||
For every city the average concentrations of elements in soils were determined. To avoid the errors related to unequal number of samples, each city was then represented by only one “averaged” sample. The statistical processing of this data allowed to calculate the average concentrations, which can be considered as the abundances of chemical elements in urban soils. |
|||
This graph illustrates the relative abundance of the chemical elements in urban soils, irregularly decreasing in proportion with the increasing [[atomic masses]]. Therefore, the evolution of organisms in this system occurs in the conditions of light elements' prevalence. It corresponds to the conditions of the evolutional development of the living matter on the Earth. The irregularity of element decreasing may be somewhat connected, as stated above, with the technogenic influence. The [[Oddo-Harkins rule]], which holds that elements with an even [[atomic number]] are more common than elements with an odd atomic number, is saved in the urban soils but with some technogenic complications. Among the considered abundances the even-atomic elements make 91.48% of the urban soils mass. As it is in the Earth's crust, elements with the 4-divisible atomic masses of leading [[isotope]] ([[oxygen]] — 16, [[silicon]] — 28, [[calcium]] — 40, [[carbon]] — 12, [[iron]] — 56) are sharply prevailing in urban soils. |
|||
In spite of significant differences between abundances of several elements in urban soils and those values calculated for the Earth's crust, the general patterns of element abundances in urban soils repeat those in the Earth's crust in a great measure. The established abundances of chemical elements in urban soils can be considered as their geochemical (ecological and geochemical) characteristic, reflecting the combined impact of technogenic and natural processes occurring during certain time period (the end of the 20th century–beginning of the 21st century). With the development of science and technology the abundances may gradually change. The rate of these changes is still poorly predictable. The abundances of chemical elements may be used during various ecological and geochemical studies. |
|||
== Human body elemental abundance == |
|||
{{main|Chemical makeup of the human body}} |
|||
{| class="wikitable" style="float:right|+ Human body elemental abundance |
|||
|- |
|||
! Element !! colspan="2"|Proportion (by mass) |
|||
|- |
|||
| [[Oxygen]] || {{bartable| 65|%}} |
|||
|- |
|||
| [[Carbon]] || {{bartable| 18|%}} |
|||
|- |
|||
| [[Hydrogen]] || {{bartable| 10|%}} |
|||
|- |
|||
| [[Nitrogen]] || {{bartable| 3|%}} |
|||
|- |
|||
| [[Calcium]] || {{bartable| 1.5|%}} |
|||
|- |
|||
| [[Phosphorus]]|| {{bartable| 1.2|%}} |
|||
|- |
|||
| [[Potassium]] || {{bartable| 0.2|%}} |
|||
|- |
|||
| [[Sulfur]] || {{bartable| 0.2|%}} |
|||
|- |
|||
| [[Chlorine]] || {{bartable| 0.2|%}} |
|||
|- |
|||
| [[Sodium]] || {{bartable| 0.1|%}} |
|||
|- |
|||
| [[Magnesium]] || {{bartable|0.05|%}} |
|||
|- |
|||
| [[Iron]] || < 0.05% || |
|||
|- |
|||
| [[Cobalt]] || < 0.05% || |
|||
|- |
|||
| [[Copper]] || < 0.05% || |
|||
|- |
|||
| [[Zinc]] || < 0.05% || |
|||
|- |
|||
| [[Iodine]] || < 0.05% || |
|||
|- |
|||
| [[Selenium]] || < 0.01% || |
|||
|} |
|||
By mass, human cells consist of 65–90% water (H<sub>2</sub>O), and a significant portion of the remainder is composed of carbon-containing organic molecules. Oxygen therefore contributes a majority of a human body's mass, followed by carbon. Almost 99% of the mass of the human body is made up of six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. The next 0.75% is made up of the next five elements: potassium, sulfur, chlorine, sodium, and magnesium. Only 17 elements are known for certain to be necessary to human life, with one additional element (fluorine) thought to be helpful for tooth enamel strength. A few more [[trace element]]s may play some role in the health of mammals. [[Boron]] and [[silicon]] are notably necessary for plants but have uncertain roles in animals. The elements aluminium and silicon, although very common in the earth's crust, are conspicuously rare in the human body.<ref>Table data from {{cite book |
|||
| last = Chang |
|||
| first = Raymond |
|||
| title = Chemistry |
|||
| edition = Ninth |
|||
| publisher = McGraw-Hill |
|||
| date = 2007 |
|||
| page = 52 |
|||
| isbn = 0-07-110595-6 }} |
|||
</ref> |
|||
'''[[Periodic table#Structure|Periodic table]] highlighting nutritional elements<ref>Ultratrace minerals. Authors: Nielsen, Forrest H. USDA, ARS Source: Modern nutrition in health and disease / editors, Maurice E. Shils ... et al. Baltimore : Williams & Wilkins, c1999., p. 283-303. Issue Date: 1999 URI: [http://hdl.handle.net/10113/46493]</ref> |
|||
[[Periodic table#Structure|Periodic table]] highlighting dietary elements |
|||
{| |
|||
! style="color: white; background-color:#006000"| <span title="Hydrogen">H</span>|| colspan="17" | ||He |
|||
|- |
|||
!bgcolor="#EEFF00"|<span title="Lithium">Li</span>||Be|| colspan="11" | ||bgcolor="#EEFF00"|<span title="Boron">B</span> ||style="color: white; background-color:#006000"|<span title="Carbon">C</span> ||style="color: white; background-color:#006000"|<span title="Nitrogen">N</span> ||style="color: white; background-color:#006000"|<span title="Oxygen">O</span> ||bgcolor="#EEFF00"|<span title="Fluorine">F</span>||Ne |
|||
|- |
|||
! bgcolor="#00B000"|<span title="Sodium">Na</span>|| bgcolor="#00B000"|<span title="Magnesium">Mg</span>|| colspan="11" | ||Al||bgcolor="#EEFF00"|<span title="Silicon">Si</span>||bgcolor="#00B000"|<span title="Phosphorus">P</span> ||bgcolor="#00B000"|<span title="Sulfur">S</span> ||bgcolor="#00B000"|<span title="Chlorine">Cl</span>||Ar |
|||
|- |
|||
! bgcolor="#00B000"|<span title="Potassium">K</span> || bgcolor="#00B000"|<span title="Calcium">Ca</span>||||Sc||Ti||bgcolor="#EEFF00"|<span title="Vanadium">V</span>||bgcolor="#EEFF00"|<span title="Chromium">Cr</span>||bgcolor="#90FF00"|<span title="Manganese">Mn</span>||bgcolor="#90FF00"|<span title="Iron">Fe</span>||bgcolor="#90FF00"|<span title="Cobalt">Co</span>||bgcolor="#90FF00"|<span title="Nickel">Ni</span>||bgcolor="#90FF00"|<span title="Copper">Cu</span>||bgcolor="#90FF00"|<span title="Zinc">Zn</span>||Ga||Ge||bgcolor="#EEFF00"|<span title="Arsenic">As</span>||bgcolor="#90FF00"|<span title="Selenium">Se</span>||bgcolor="#90FF00"|<span title="Bromine">Br</span>||Kr |
|||
|- |
|||
!|Rb||bgcolor="EEFF00"|<span title="Strontium">Sr</span>||||Y ||Zr||Nb||bgcolor="#90FF00"|<span title="Molybdenum">Mo</span>||Tc||Ru||Rh||Pd||Ag||Cd||In||Sn||Sb||Te||bgcolor="#90FF00"|<span title="Iodine">I</span> ||Xe |
|||
|- |
|||
!Cs||Ba||* ||Lu||Hf||Ta||W ||Re||Os||Ir||Pt||Au||Hg||Tl||Pb||Bi||Po||At||Rn |
|||
|- |
|||
!Fr||Ra||** ||Lr||Rf||Db||Sg||Bh||Hs||Mt||Ds||Rg||Cn||Uut||Fl||Uup||Lv||Uus||Uuo|| |
|||
|- |
|||
| colspan="19"| |
|||
|- |
|||
! colspan="3"| ||* ||La||Ce||Pr||Nd||Pm||Sm||Eu||Gd||Tb||Dy||Ho||Er||Tm||Yb|| |
|||
|- |
|||
! colspan="3"| ||**||Ac||Th||Pa||U ||Np||Pu||Am||Cm||Bk||Cf||Es||Fm||Md||No|| |
|||
|} |
|||
{| |
|||
| style="color: white; background-color:#006000"|The four organic basic elements |
|||
| bgcolor="#00B000"|Quantity elements |
|||
| bgcolor="#90FF00"|Essential [[trace element]]s |
|||
| bgcolor="#EEFF00"|Possible structural or functional role in mammals |
|||
|} |
|||
== See also == |
|||
*[[Abundances of the elements (data page)]] |
|||
*[[Natural abundance]] (isotopic abundance) |
|||
*[[Primordial nuclide]] |
|||
--> |
|||
== Referensi == |
== Referensi == |
||
{{reflist}} |
{{reflist|30em}} |
||
=== Bacaan lanjutan === |
|||
* http://geopubs.wr.usgs.gov/fact-sheet/fs087-02/ |
|||
* http://imagine.gsfc.nasa.gov/docs/dict_ei.html |
|||
== Pranala luar == |
== Pranala luar == |
||
{{portal|kimia}} |
|||
* [http://www.science.co.il/PTelements.asp?s=Earth List of elements in order of abundance in the Earth's crust] (only correct for the twenty most common elements) |
|||
*[http://www.chem.ualberta.ca/~massspec/atomic_mass_abund.pdf Tabel Massa Isotop dan Kelimpahan Alami] dari Universitas Alberta, Kanada |
|||
* [http://web.archive.org/web/20060901133923/http://www.astro.wesleyan.edu/~bill/courses/astr231/wes_only/element_abundances.pdf Cosmic abundance of the elements and nucleosynthesis] |
|||
*[http://periodictable.com/Properties/A/IsotopeAbundances.html Kelimpahan isotop seluruh unsur di alam] |
|||
* [http://www.webelements.com/periodicity/ webelements.com] Lists of elemental abundances for the Universe, Sun, meteorites, Earth, ocean, streamwater |
|||
{{Compact periodic table}} |
|||
<!--{{DEFAULTSORT:Abundance Of The Chemical Elements}} |
|||
[[Category:Astrochemistry]] |
|||
[[Category:Properties of chemical elements]] --> |
|||
[[Kategori:Sifat kimia]] |
|||
{{kimia-stub}} |
|||
[[Kategori:Isotop]] |
|||
{{Uncategorized stub|date=April 2016}} |
|||
Revisi per 12 Januari 2017 14.44
Kelimpahan alami unsur (natural abundance, NA) dalam ilmu kimia mengacu kepada kelimpahan isotop-isotop suatu unsur kimia yang secara alami dapat ditemukan di satu planet. Massa atom (rata-rata bobot) isotop-isotop ini adalah massa atom satu unsur kimia seperti yang ditulis dalam tabel periodik. Kelimpahan isotop berbeda dari planet ke planet, bahkan juga dari tempat ke tempat di Bumi, namun tetap konstan setiap saat.
Sebagai contoh, uranium mempunyai tiga isotop yang dihasilkan secara alami: 238U, 235U dan 234U. Kelimpahan alami masing-masing berkisar antara 99.2739 - 99.2752%, 0.7198 - 0.7202%, dan 0.0050 - 0.0059%.[1] Apabila 100,000 atom uranium dianalisis, seseorang bisa menebak ada sekitar 99,275 atom 238U, 720 atom 235U, dan hanya 5 atau 6 atom 234U. Ini karena 238U jauh lebih stabil daripada 235U atau 234U, dan hal ini dapat dibuktikan dengan waktu paruh tiap isotop: 4.4468×109 tahun untuk 238U berbanding dengan 7.038×108 bagi 235U dan 245,500 tahun untuk 234U. Namun, kelimpahan alami suatu isotop juga dipengaruhi oleh kemungkinan pembuatannnya dalam sintesis nuklir (seperti dalam kasus samarium; 147Sm dan 148Sm yang bentuk radioaktifnya lebih banyak di alam daripada 144Sm yang stabil) dan juga oleh produksi suatu isotop melalui isotop alami radioaktif (seperti dalam kasus isotop timbal radiogenik).
Penyimpangan dari kelimpahan alami
Dari studi tentang Matahari dan meteorit-meteorit primitif, kini telah diketahui bahwa sistem tata surya awalnya memiliki komposisi isotop yang hampir homogen. Deviasi dari rata-rata galaktik (yang berubah) yang disampel secara lokal sekitar waktu ketika pembakaran inti Matahari dimulai, dapat secara umum diperhitungkan dengan pemeringkatan massa dan sejumlah terbatas peluruhan inti dan proses-proses transmutasi.[2] Ada juga bukti penyuntikan isotop-isotop berjangka panjang pendek (kini hilang) dari ledakan supernova terdekat yang mungkin telah memulai runtuhnya nebula matahari.[3] Jadi, penyimpangan dari kelimpahan alami di bumi biasanya dinyatakan dalam bagian per seribu (permil atau ‰) karena kurang dari satu persen (%).
Satu pengecualian untuk ini terletak di dalam butiran pramatahari yang ditemukan dalam meteorit primitif. Ia telah melewati proses penghomogenan, dan sering membawa tanda inti proses-proses sintesis nuklir tertentu yang membentuk unsur-unsur butiran ini.[4] Dalam materi ini, deviasi dari kelimpahan alami kadangkala diukur dalam faktor 100.
Lihat pula
Referensi
- ^ Uranium Isotopes, diakses tanggal 14 Maret 2012
- ^ Robert N. Clayton (1978) Isotopic anomalies in the early solar system, Annual Review of Nuclear and Particle Science 28:501-522.
- ^ Ernst Zinner (2003) An isotopic view of the early solar system, Science 300:5617, 265-267.
- ^ Ernst Zinner (1998) Stellar nucleosynthesis and the isotopic composition of presolar grains from primitive meteorites, Annual Review of Earth and Planetary Sciences 26:147-188.
Pranala luar
- Tabel Massa Isotop dan Kelimpahan Alami dari Universitas Alberta, Kanada
- Kelimpahan isotop seluruh unsur di alam

